The limited success of tissue characterization in medical ultrasound is largely due to the presence of an inhomogeneous soft tissue medium between the tissue structure of interest and the insonifying ultrasound transducer. Phase aberration distorts the acoustic wavefront as it propagates through the inhomogeneous tissue, which has a large and unpredictable effect on the output voltage of the receiving transducer. In addition, the output voltage is affected by attenuation and multiple transmission paths through the soft tissue. The broadband integrated backscatter (IBS) measures the normalized energy of the RF backscatter signal from a given sample volume. The rationale behind using IBS is that the integration operation removes much of the random fluctuation in the RF signal while still preserving the spatial resolution. IBS has been used successfully for tissue characterization, such as for characterizing normal myocardium from ischemic myocardium, and for a number of NDE applications.
We have been investigating the use of blood as a reference backscatter (see Figure 1) for the purpose of obtaining integrated backscatter "signatures" of atherosclerotic plaques in an absolute sense, with the goal of being able to differentiate between stable and vulnerable atherosclerotic plaques. The diagnostic utility of using backscatter level to determine plaque type is based on the dramatic spread in backscatter level across different plaque types and relative to normal arterial vessel wall. Given that the backscatter (Doppler) ultrasound signal from flowing blood is stochastic in nature, it is important to be able to estimate the IBS variance, or alternatively perform the measurements so that an acceptably low variance is attained.
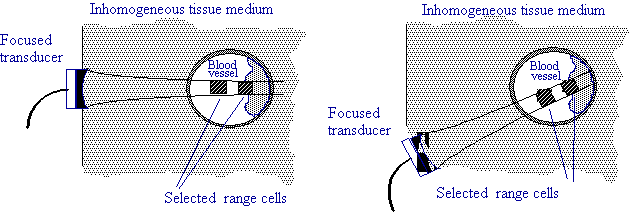
Figure 1. Using integrated backscatter (IBS) as a normalizing factor for plaque/wall echoes.
The primary purpose of this work is to establish a theoretical framework that allows calculation of the variance of the IBS estimate, given the stochastic properties of the backscatter signals from flowing blood and the parameters of the experimental data acquisition. From this, the achievable accuracy of the integrated backscatter level from blood for a given experimental situation can be determined. The work formulates further how the measurement parameters should be specified so that an IBS estimate of moving blood with an appropriately low variance is ensured or, alternatively, a specified accuracy of the tissue IBS estimate is obtained. Through modeling, simulations and experiments (see Figure 2) we show how the variance of the IBS estimate of the blood backscatter signal can be quantified and reduced to a specified tolerable level. (See pictures from our lab here and here.)
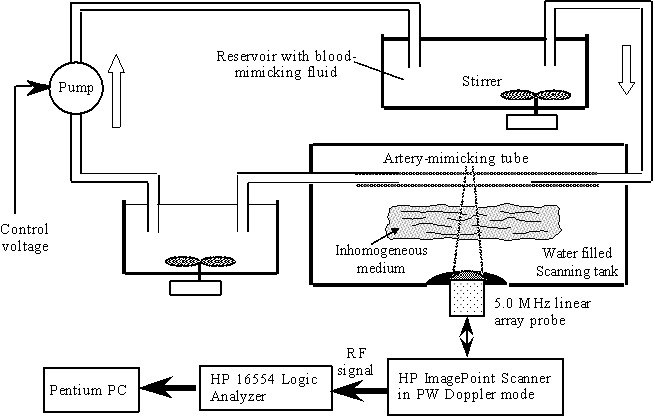
Figure 2. Block diagram of the experimental set-up. The closed reservoir removes rapid pressure fluctuation from the flow. The pump is set to produce a constant flow rate.
The ultimate goal of this research is to develop a screening tool for stroke risk. Stroke is the third largest killer, after heart disease and cancer. In the US, about 700,000 people suffer strokes each year. About 500,000 of these are first attacks and 200,000 are recurrent attacks. Stroke is estimated to cost $30 to $40 billion per year. As many as 60 - 70% of all strokes are caused by emboli from “vulnerable” plaques in the carotid artery (see Figure 3). Treatment regimens are available, such as surgical removal of plaque material (carotid endarterectomy) or carotid artery stenting, a recent procedure where an expandable metal-mesh tube, called a stent, is inserted into the carotid artery to increase blood flow.
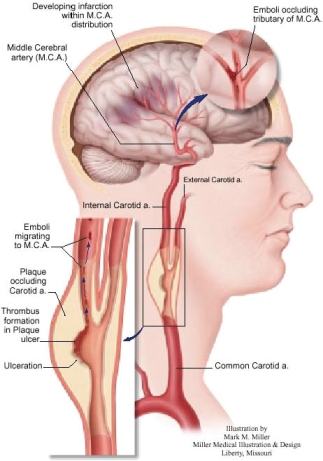
Figure 3. An atherosclerotic plaque occluding the carotid artery.
Atherosclerotic lesions contain a combination of fatty, fibrofatty, fibrotic and calcified materials, each with different ultrasound backscatter characteristics, and there appears to be a strong correlation between plaque composition and vulnerability/stability of plaque. Thus, morphology and composition of atherosclerotic plaque is predictive of stroke risk, and may be detected ultrasonically. The major challenge with ultrasound-based plaque classification is the phase aberration of the ultrasound beam caused by the soft tissue between the skin surface and plaque region of interest, which modifies echoes from a given vessel wall and plaque in an unpredictable way. However, this may be overcome by using a reference backscatterer in the immediate vicinity of the plaque or vessel wall: a volume of flowing arterial blood inside the lumen of the carotid artery, adjacent to the plaque region of interest. Arterial blood, however, must be treated as a stochastic scatterer. In our approach, the Integrated backscatter (IBS) of the plaque or wall echoes is normalized with the IBS of an adjacent volume of arterial blood.